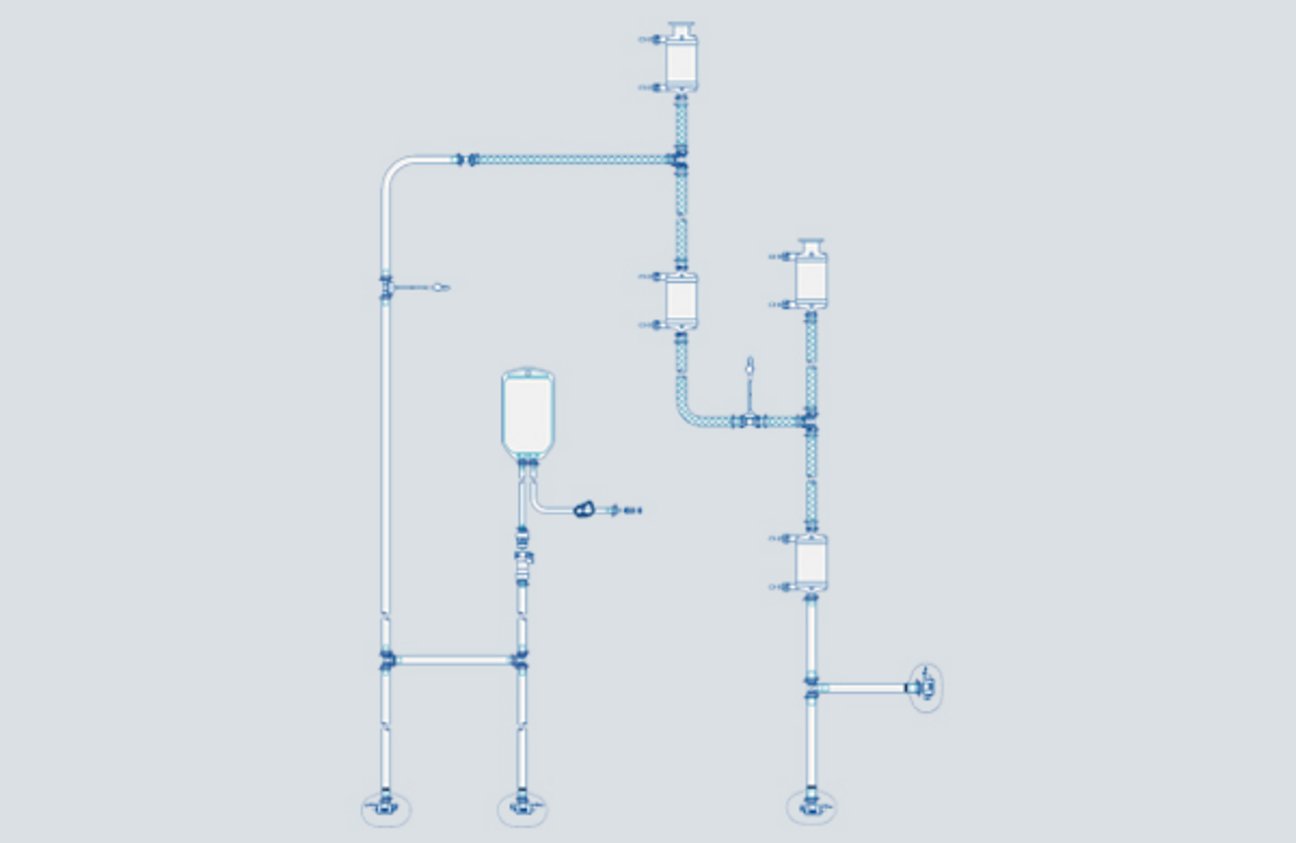
PUPSIT Assemblies
PUPSIT determines the integrity of the sterilizing-grade filter and assembly. PUPSIT is performed to help ensure that the filter has not been damaged during shipping, installation, and sterilization prior to use. A damaged filter could result in a non-sterile product, which can ultimately affect drug safety. Masterfilter offers a range of pre-designed and pre-sterile PUPSIT Assemblies which are available in a wide variety of configurations.
Features
- Full line of single-use products including sterilizing-grade filters, storage bags, tubing, aseptic connectors, fittings, pressure gauge, in-line pressure sensors and monitor, etc. Meet almost all needs for sterile filtration in final filling
- Pre-designed and self-produced assemblies, save at least 50% waiting times
- A complete listing of supporting documents, including validation guide, extractable data, system design guide, user manual, application data, etc.
Quality Assurance
- ISO Class 4.8 clean area assemble (equivalent to GMP Class A cleanliness)
- ISO 9001:2015 Quality Management System
- USP <87> Biological Reactivity Test, In Vitro
- USP <88> Class VI plastics Biological Reactivity Test, In Vivo
- Bacterial endotoxin meets WFI requirements (< 0.25 EU/ml)
- Particles matter meet the requirements in CP and USP <788> for large-volume parenterals
- Gamma radiation dose validation according to ISO 11137, sterile packaging