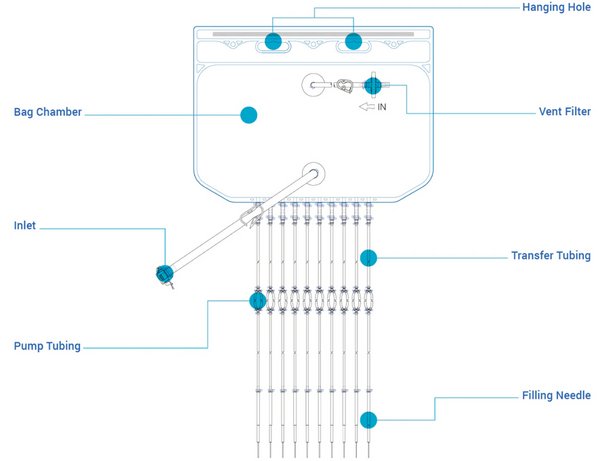
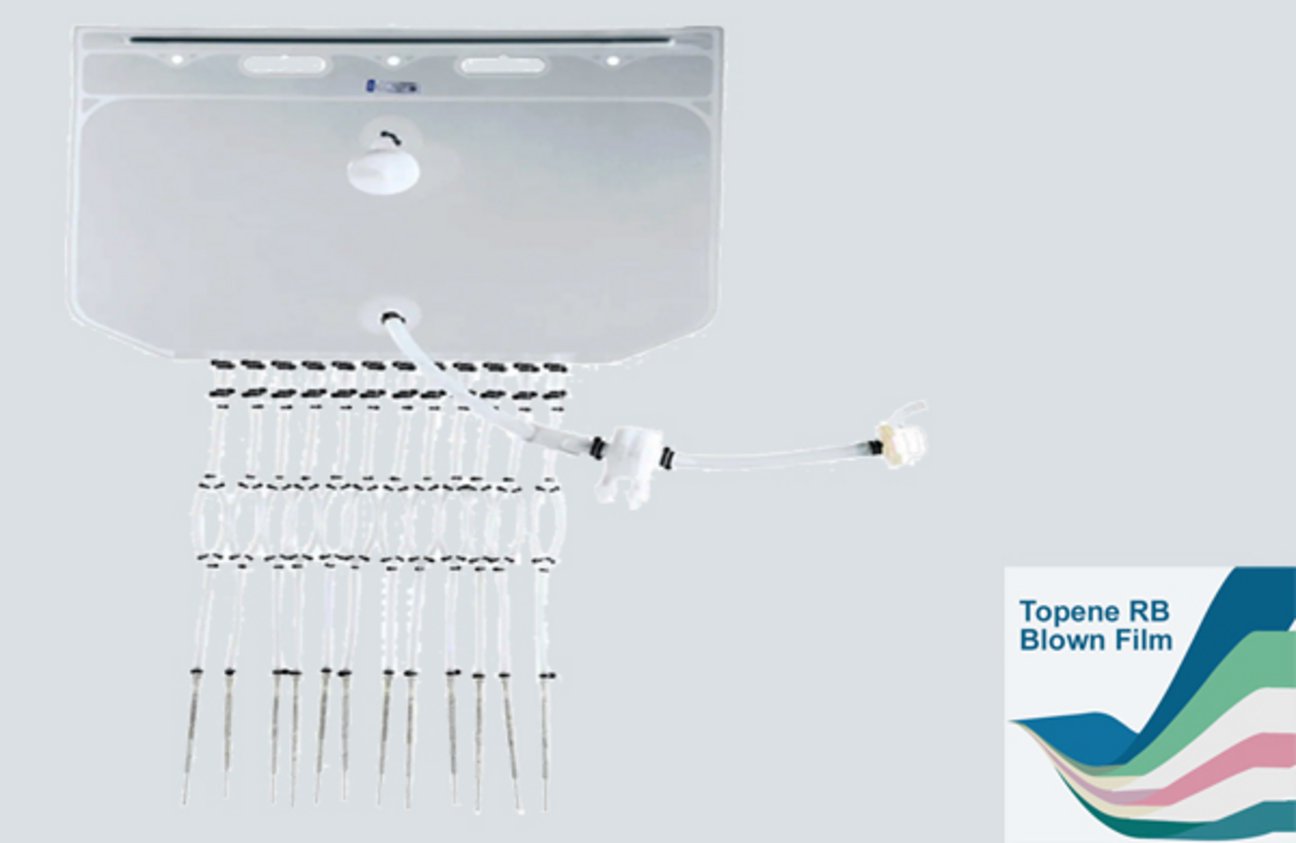
Final Filling Assemblies
Masterfilter Single-Use Final Filling Assemblies is a pre-assembled, pre-sterilized aseptic transfer production unit, including liquid storage bags, sterile filtration, single-use filling bags, filling tubing, and filling needles. Single-use filling assemblies are designed for the aseptic processing and transfer of biopharmaceutical fluids. They represent a critical advancement in modern drug manufacturing, replacing traditional stainless-steel vessels to enhance efficiency, safety, and flexibility.
Features
- Innovative Topene™ RB blown film, reducing the contamination of particles
- Sterilized by 25-45 kGy gamma irradiation
- Designed with boat ports for ultra-low residual liquid
- Validation services are available for extractables and leachables, chemical compatibility
- Customized assemblies
Quality Assurance
- ISO 9001: 2015 quality management system
- Filling bags are manufactured exclusively in a dedicated ISO 4.8 cleanroom. Fittings entering through transfer window. Personnel entering through air shower room.
- 100% bag integrity test
- ADCF raw material meets the requirement of FDA 21 CFR 177-182 indirect food additives
- Meets the requirement of ISO 11137 sterilization validation
- Meets the requirement of USP <87> in Vitro Biological Reactivity Test
- Meets the requirement of USP <88> Biological Reactivity Test, in Vivo for Class VI plastics
- Aqueous extraction contains < 0.25 EU/ml as determined by Limulus Amebocyte Lysate (LAL), USP <85>
Applications
- Antibodies
- Vaccine
- Gene therapy
- Etc.
Configuration Options